The puzzle
|
The dominant paradigm in TB research has rested upon years of evidence showing that cell-mediated immunity, and specifically effector CD4 T cell activity IFNg production, accelerates protective immunity and correlates with reduced disease burden. Unfortunately, attempts to target such T cells for prophylactic vaccination have not elicited protection above BCG, the only currently available vaccine. Work from our lab shows that IFNg-producing Th1 cells (characterized by the transcription factor T-bet) have relatively limited expansion and differentiation potential (Künzli et al Sci Immunol; Swarnalekha et al Sci Immunol). Consistent with this, Mtb specific Th1 effectors in the lung are less proliferative and fail to control Mtb following transfer/challenge in mice. These observations highlight the insufficiency of targeting Th1 effectors in isolation and underscore the importance considering additional - potentially synergistic - mechanisms of protection.
|
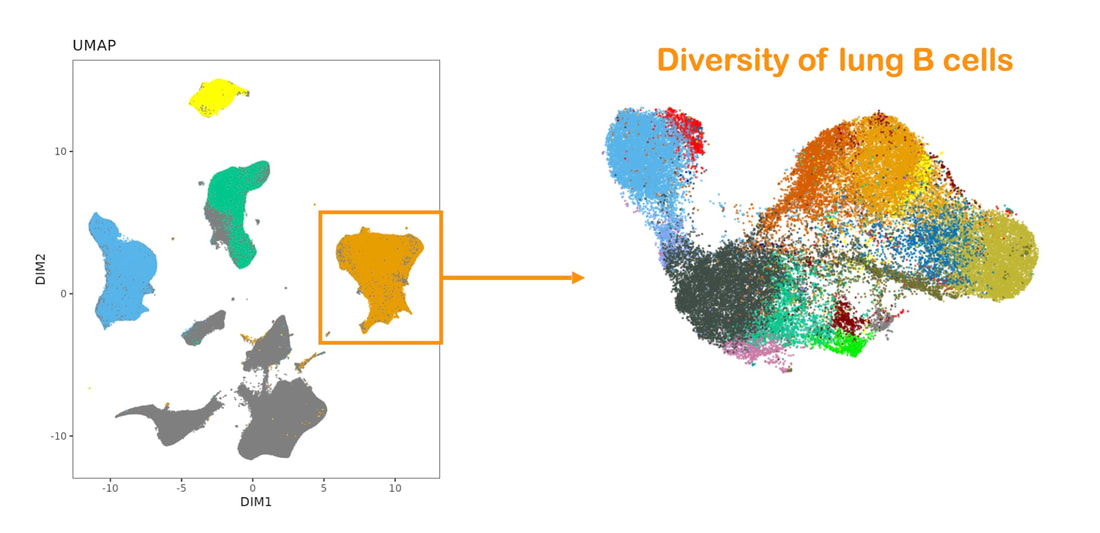
Shifting the paradigm to include humoral immunity: The immune system employs many strategies when dealing with pathogens, and a useful distinction can be drawn between 1) minimization of pathology in an infected host; 2) sterilization of an existing infection; and 3) resistance to infection. Strategy 1, the management of pathology, is clearly important in the case of TB as most infected people remain only latently infected. Cell-mediated immunity is largely responsible for strategy 2, as it implies cells killing either internalized microbes or infected or aberrant cells. A prophylactic vaccine typically strives to implement strategy 3 by preventing the infection of host cells by the pathogen, typically by eliciting protective antibodies. Nevertheless, relevant studies on antibodies and B cells during TB are scarce. Importantly, infection with Mtb, and thus the opportunity for resistance, occurs not only during exposure to inhaled bacteria but also when a latently infected person experiences an activation of disease leading to dissemination of bacteria into the extracellular space. Because the infectious inoculum of Mtb is typically very small, 1-36 bacilli in the case of primary exposure, an effective humoral response in the lung could be the difference between infection and resistance.
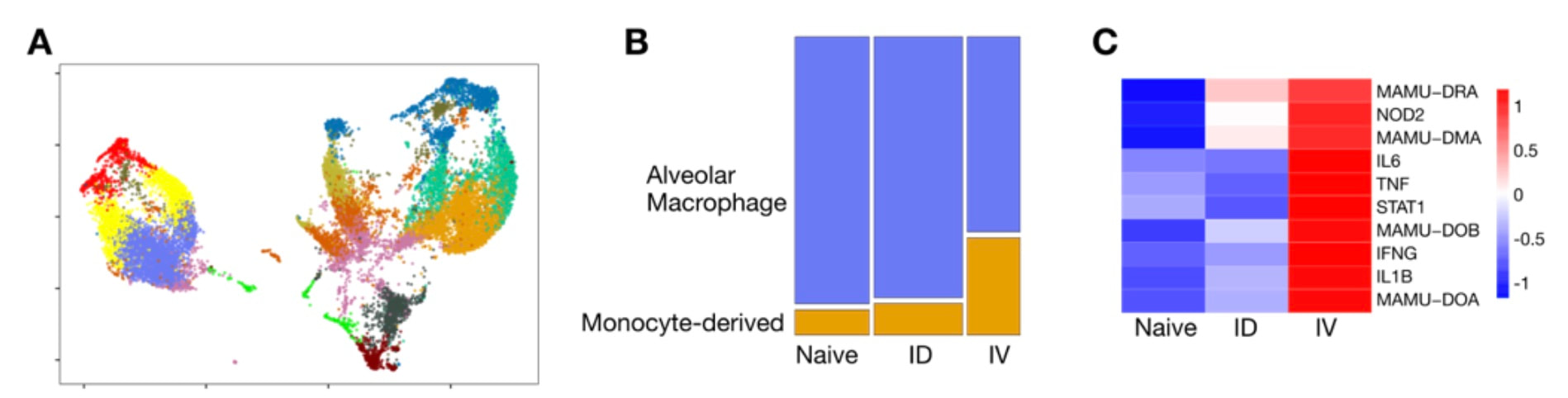
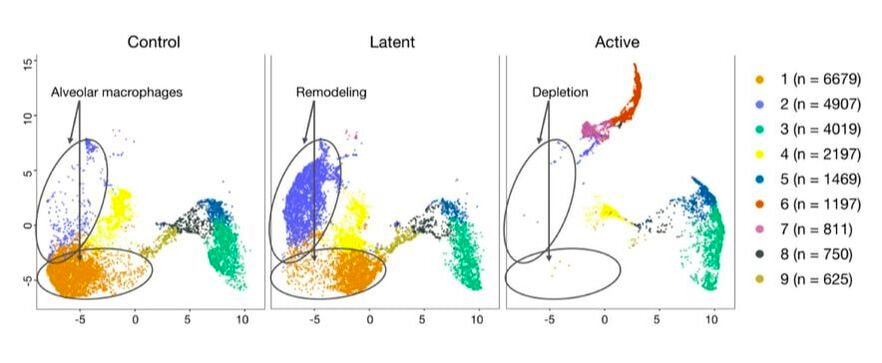
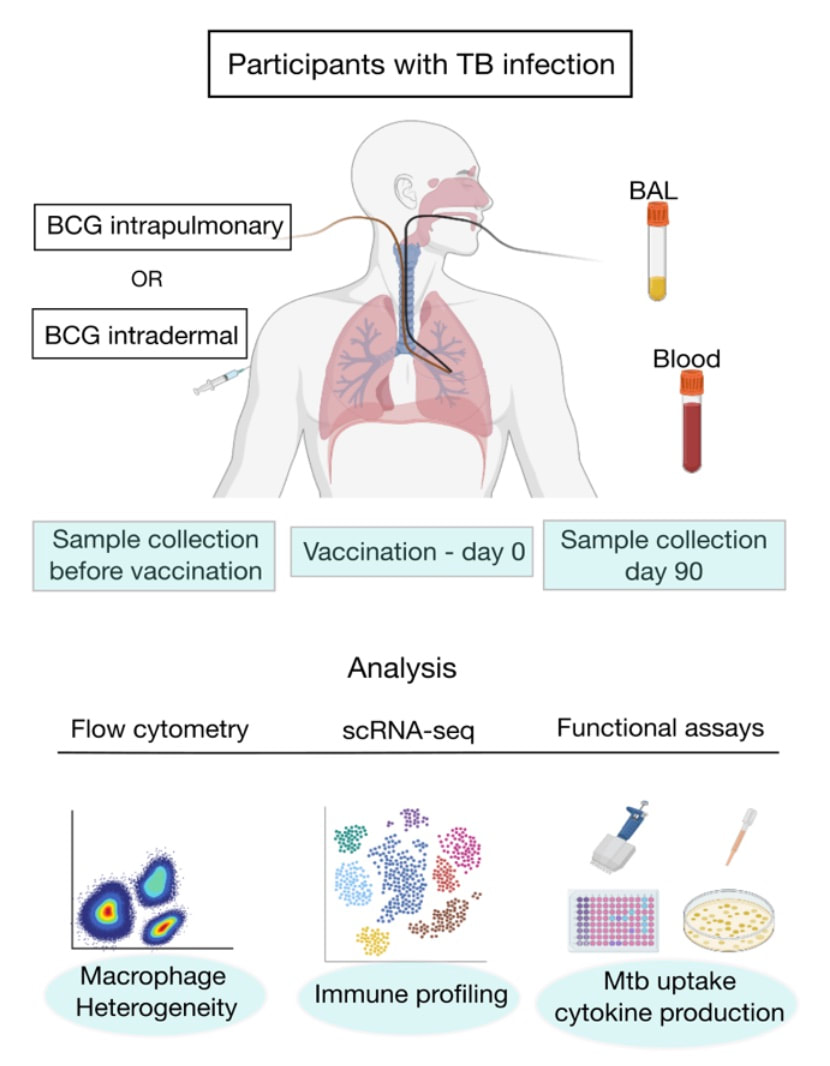
Macrophages in the mix: The role of airway macrophages as the initial site of Mtb infection is well established, and unbiased single cell approaches have recently highlighted the phenotypic and functional diversification of this compartment. Our data and secondary analyses indicate that mycobacterial infection induces significant alveolar macrophage depletion in humans, macaques and mice; the level of depletion appears to depend on the route of immunization and/or bacterial load. We are investigating the hypothesis that AM depletion removes a major niche for mycobacterial growth which induces the generation of heterogeneous interstitial macrophages that are resistant to infection. By combining targeted interventions in a mouse model, a unique model of mucosal BCG vaccination in humans and the latest single cell technologies, we are dissecting the dynamics, function and intercellular niche of diverse macrophage populations following mycobacterial exposure. |
UPDATE: This collaborative project with Professor Keertan Dheda at the University of Cape Town, has recently been funded by the Swiss National Science Foundation! We are very excited to join forces with the Dheda group, maximizing the potential of controlled human infection models to inform our understanding and treatment of TB. See HERE for an update on TB-CHIM.